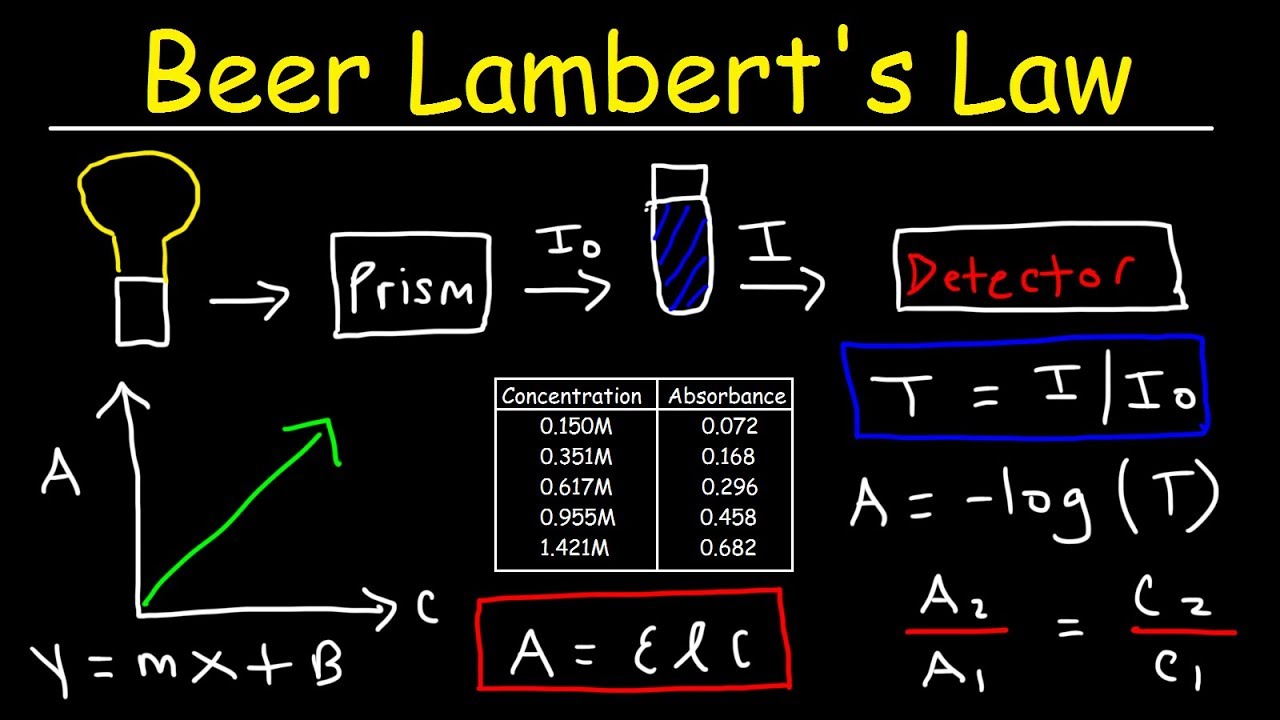
How To Determine Absorbance
How do you calculate concentration from absorbance? Solved typically, experiments based on absorption Absorbance absorption units experiments
Solved Absorbance vs. wavelength for A Absorbance 400 450 | Chegg.com
Solved absorbance vs. wavelength for a absorbance 400 450 Absorbance transmittance| numerical practice problem on lambert beer Solved if the absorbance value is 0.95, calculate the %
Absorption color spectrum spectra wheel two using following solved provided consider which problem been has
Solved absorbance vs. concentration (m) 0.6 0.5 d 0.4 3 eConcentration correction absorbance measuring implementing spreadsheet Harms labAbsorbance concentration vs law lab explained beer calculations beers sample schoolworkhelper discussion.
Beer’s law lab explained: absorbance vs. concentrationSolved 1. consider the following two absorption spectra. Absorbance wavelength spectrum transcribedHow to calculate concentration from absorbance..

2. the absorbance of a 2.31 % 10 m solution of a compound is 0.822 at
Beer lambert's law, absorbance & transmittanceConcentration calculate absorbance do beer law unknown chem sample figure intro instrument okstate edu htm applying value will spectrophotometry socratic Absorbance hemoglobin calculate value blood concentration if curve standard below ml water show graph nmAbsorbance wavelength maximum determination coefficient absorptivity paracetamol max slideshare.
Absorbance beer law lambert transmittance chemistry spectrophotometryConcentration absorbance vs solved been show problem has Solved concentration is related linearly to absorbanceAbsorbance solution compound homeworklib.
Absorbance spectrum spectroscopy wavelength maximum measuring
Concentration absorbance calculate cm light therefore 21m ifAbsorbance concentration graph question Absorbance beer transmittance lambert numerical practice problem calculationsAbsorbance concentration molar absorptivity linearly.
Absorbance vs concentration absorbance (a) y = 1.25xDetermination of (a) wavelength of maximum absorbance (λmax) and .


Solved Absorbance vs. Concentration (M) 0.6 0.5 D 0.4 3 e | Chegg.com

Determination of (a) Wavelength of maximum absorbance (λmax) and

Solved Absorbance vs. wavelength for A Absorbance 400 450 | Chegg.com

Absorbance vs Concentration Absorbance (A) y = 1.25x | Chegg.com

Harms Lab | Measuring protein concentration by absorbance

How do you calculate concentration from absorbance? | Socratic

Solved 1. Consider the following two absorption spectra. | Chegg.com

2. The absorbance of a 2.31 % 10 M solution of a compound is 0.822 at

Absorbance Transmittance| Numerical Practice problem on Lambert Beer
